Abstract
Decentralized Clinical Trials or “direct-to-participant trials” or “virtual” studies are characterized by less dependence on traditional research facilities or specialist intermediaries for data collection. Instead, it’s about looking at areas where information technology can enable sponsors and CROs to take a hybrid approach to clinical trial design, providing an alternative to a site-centric, inflexible system which often results in a high patient drop-out.
DCTs leverage “virtual” tools, such as telemedicine, Internet-of-Things, home visits, subject-driven virtual healthcare interfaces, and direct delivery of study drugs and materials to subjects’ homes. In a fully decentralized clinical trial, subject recruitment, delivery and administration of study medication, and acquisition of trial outcomes data all proceed without involving person-to-person physical contact between the research team and the subject.
Why Site-Centered Clinical Trials are Becoming Unfeasible
As a result of disruptive challenges in the post-Covid world, there has been a necessity to identify and incorporate new solutions in the world of clinical trials. Just like COVID-19 has created a barrier in the healthcare sector, where patients are reluctant to visit healthcare providers like hospitals and clinics due to the fear of getting infected. Enrollment of subjects participating in clinical trials has been affected, as clinical trials involve visiting sites for the subjects, and interacting with investigators and site staff, which also magnifies the risk of getting infected.
To mitigate this problem, the concept of decentralized clinical trials has rapidly evolved, to reduce patient burden, increase patient enrollment and retention, and preserve the quality of life, while also increasing the efficiency of trial logistics.
Therefore, the clinical trial environment is moving toward remote collection and analysis of data, transitioning from the classic site-centric model to one that is more patient-centric. Information Technology has a key role to play in the patient-centric model where processes like online scheduling of subject visits, tele-visits, remote model of site monitoring and e-based clinical trial supply chain management has become the norm rather than the alternative.
Ensuring the Success of Decentralized Clinical Trials through Proper Use of Information Technology
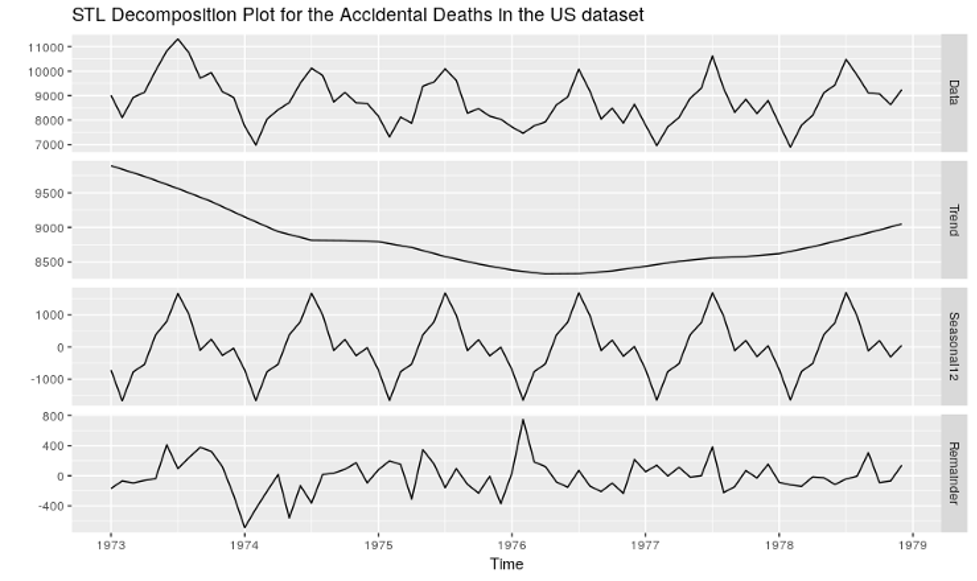
To ensure that the Decentralized Clinical Trials are conducted effectively, several established software systems, which used to work in silos in conventional clinical trials, have to work in an integrated manner.
When the clinical trial is getting initiated at a site, subject enrollment has to be initiated by a site-facing portal, which utilizes the sponsor or CRO’s clinical data repository to get subject referrals, and then send out invitations to these potential participants through automated emails. The next step in the process has to be initiated by the participant, by using the link embedded in these emails to get themselves registered in the Participant Portal System for the specific study and site.
In order to go through the screening phase, they have to upload their medical documents in the portal, which will be readily available to the site staff who will assess these documents to determine whether the potential participant fits the study’s inclusion criteria. Those prospective participants who pass screening will be informed through the Participant Portal System to get themselves ready for the consent visit. But the consent visit will not require the participant to physically visit the site, but instead to join a tele-visit through the Participant Portal System where they will meet with the principal investigator and other site staff. This will require a video conferencing software to be integrated with both the site portal and the Participant Portal. Further, the Consent Management System must work in tandem, so that consent can be provided by the participant during the tele-visit using a computer or smartphone device, and it can be counter-signed by the principal investigator.
As the participant’s status moves from “screened” to "consented”, the randomization system comes into play, which will collect stratification data like age, gender and ethnicity from the participant portal and use the trial design algorithm to assign the participant to a specific trial arm of the study. The clinical logistics system will pick up this data to determine which investigational product kit needs to be supplied to the participant. As the first post-randomization dosage visit for the participant gets scheduled, the kit will be shipped to the participant.
The dosage trial tele-visit will involve the site staff from the site and the participant, in which during the video conference, the participant will self-administer the dosage with the help of a personal caregiver or preferred local healthcare professional (information regarding the latter will have to be provided by the participant using the participant portal in advance).
One of the major advantages of the tele-visit model over the physical visit model is that the entire visit, including the exact start time and end time of the visit will be recorded and saved in the integrated DCT database. Site monitors can remotely access these recordings to assess no protocol deviations happen with respect to dosage administration or visit schedule, without the risk of the data getting manipulated.
Any sample collection that needs to be done for the participant is also done during the tele-visit and the sample can be directly shipped to the laboratory by the participant, reducing the burden on the sitestaff as they no longer need to ensure that samples for each participant have to be shipped by them to 3rd party laboratories on time, and they can utilize their time for more value-added activities like assessment of e-Diaries submitted by participants through the participant portal to gauge response of IP dosage and potential adverse events. Of course, a prerequisite to this would be integrating the sponsor’s lectronic atient Reported Outcome System with the participant portal to ensure regular and timely availability of e-Diaries to the participant with necessary automated reminders.
The Integrated DCT Model
Conclusion
In the pre-covid world, the main factors behind low participant enrollment and high participant drop-outs were attributed to lack of engagement on part of site staff, which resulted from complexity of trial eligibility criteria, resource constrains facing supporting staff and extra workload on investigators.
In post-covid world site accessibility has been added to this list of constraints, increasing the risk of prolongated clinical trials, and thus causing a lot of loss to the sponsor and medical science. But every new problem also provides a new opportunity, and re-modelling the clinical trial value chain using the DCT approach can actually have a positive impact by accelerating patient recruitment, increasing participant diversity and retention, improved reliability and accuracy of data and most importantly making the participant more knowledgeable, informed and engaged as a result of autonomy enabled by information technology.
References:
- Decentralizing Clinical Trials: The White Paper - https://www.acrohealth.org/dctwhitepaper/
- Why Decentralisation Is the Future of Clinical Trials - https://mdgroup.com/blog/why-decentralisation-is-the-future-of-clinical-trials/
- JACC Journals- Decentralized Clinical Trials: The Future of Medical Product Development? - https://www.jacc.org/doi/full/10.1016/j.jacbts.2021.01.011
- Conceptualization of a medical data collection system intended for decentralized clinical trials - https://lup.lub.lu.se/student-papers/record/9082970/file/9083136.pdf
About Encora
Fast-growing tech companies partner with Encora to outsource product development and drive growth. Contact us to learn more about our software engineering capabilities.